The ANGPTL3-targeting agent, SHR-1918, significantly reduced LDL-C, TG, apoB and other atherogenic lipids in patients with homozygous familial hypercholesterolemia (HoFH) in a single arm, Phase 2 non-randomised study carried out at eight sites in China.
Fish oil supplements reduce serious CV events in patients on haemodialysis
Patients undergoing maintenance haemodialysis who take daily n-3 fatty acid supplements can reduce their rates of serious cardiovascular (CV) events, according to recently published results of the PISCES trial.
2025: A Year of Progress in Triglyceride Research
Over the past year, progress in the field of TG research has continued to gain momentum, with important developments across science, clinical care, and policy reported on Triglyceride Forum. Regulatory Developments US and European regulatory approvals for novel TG lowering agents as an adjunct to diet expanded therapeutic opportunities for patients with familial chylomicronaemia syndrome. […]
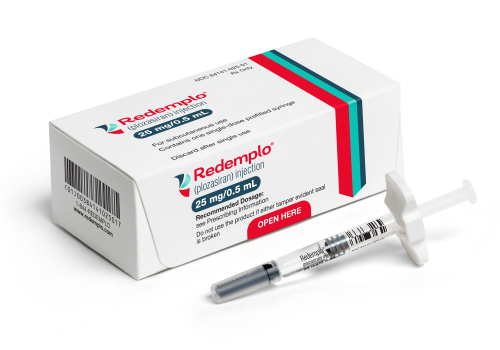
Redemplo (plozasiran) receives US approval for FCS
The US Food and Drug Administration (FDA) has approved Redemplo (plozasiran) as an adjunct to diet to reduce triglycerides in adults with familial chylomicronaemia syndrome (FCS). The apoC3 siRNA will be available in the USA before the end of the year.
APOC3 gene-silencing agents predicted to reduce heart disease risk by 25%
Novel APOC3 gene-silencing agents currently in development could reduce coronary heart disease (CHD) risk by approximately 25% during a 5-year outcomes trial. This conclusion is based on results from a polygenic score (PGS)-based model to investigate if the degree of triglyceride rich lipoprotein (TRL)/remnant reduction seen with APOC3 gene-silencing would lead to a meaningful reduction […]
Growing support for benefits of remnant reduction
Reducing remnant cholesterol (RC) levels by at least 50% in individuals with levels >1 mmol/L (39 mg/dL) may have substantial benefits in reducing their risk of atherosclerotic cardiovascular disease (ASCVD).