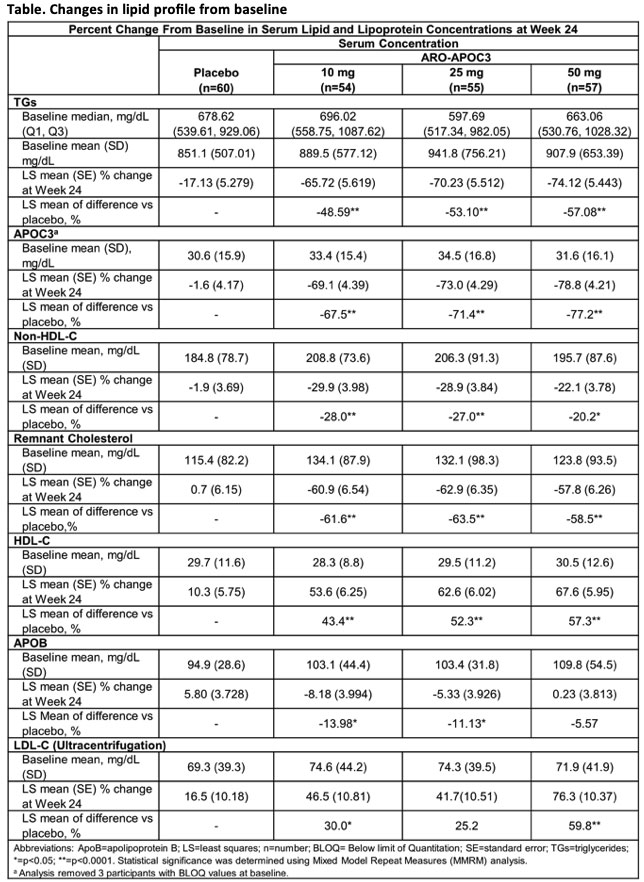
Interim results from the SHASTA-2 study of the investigational apoC3 targeting RNAi agent, plozasiran (ARO-APOC3) in patients with severe hypertriglyceridaemia (HTG) have shown a reduction in TG levels below the risk threshold for acute pancreatitis (<500 mg/dL) at 24 weeks follow up.
In the Phase 2b study, 229 patients with TG levels >500 mg/dL were randomised to receive plozasiran 10 mg, 25 mg, or 50 mg s.c. or matched placebo once every 12 weeks on day 1 and at week 12. Median baseline serum TG ranged from 598 mg/dL to 696 mg/dL. At week 24, there was a significant reduction in mean serum apoC3 levels of up to 79% with plozasiran compared to placebo (p=0.007) (Table). Serum TG was significantly reduced by 66 to 74% (p<0.0001). Over 90% of study participants in the plozasiran 25 mg and 50 mg dose groups achieved TGs <500 mg/dL as early as week 4 that was sustained through week 24, and 50% to 72% achieved normalisation of TGs (<150 mg/dL) from week 4 through week 24. Mean non-HDL-C was reduced by up to 30% and mean serum HDL-C increased by up to 68%.
The most frequently reported treatment emergent adverse events (TEAEs) were COVID-19 infections, headache, and worsening glycaemic control. Serious TEAEs were not related to plozasiran and were resolved without sequelae, except two patients with malignancies.
Professor Daniel Gaudet (University of Montreal, Canada) concluded that, based on the SHASTA-2 results, RNAi-mediated silencing of hepatic apoC3 expression via plozasiran is a promising potential treatment for people with severe HTG.
Reference
Gaudet D. ARO-APOC3, an Investigational RNAi Therapeutic, Silences APOC3 and Reduces Triglycerides to Near Normal Levels in Patients With Severe Hypertriglyceridemia: SHASTA-2 Study Results. Presented at American Heart Association Scientific Sessions 2023 (11-13 November, Philadelphia, USA) PR.AOS.549 – Targeted Lipid Lowering via Novel Pathways Session, 302.